In this short blog, we are going to look at one of our mass flow controller calibration reports and discuss some of the terms that you will see. There is good information at the bottom of these reports, so let’s jump in and take a closer look…
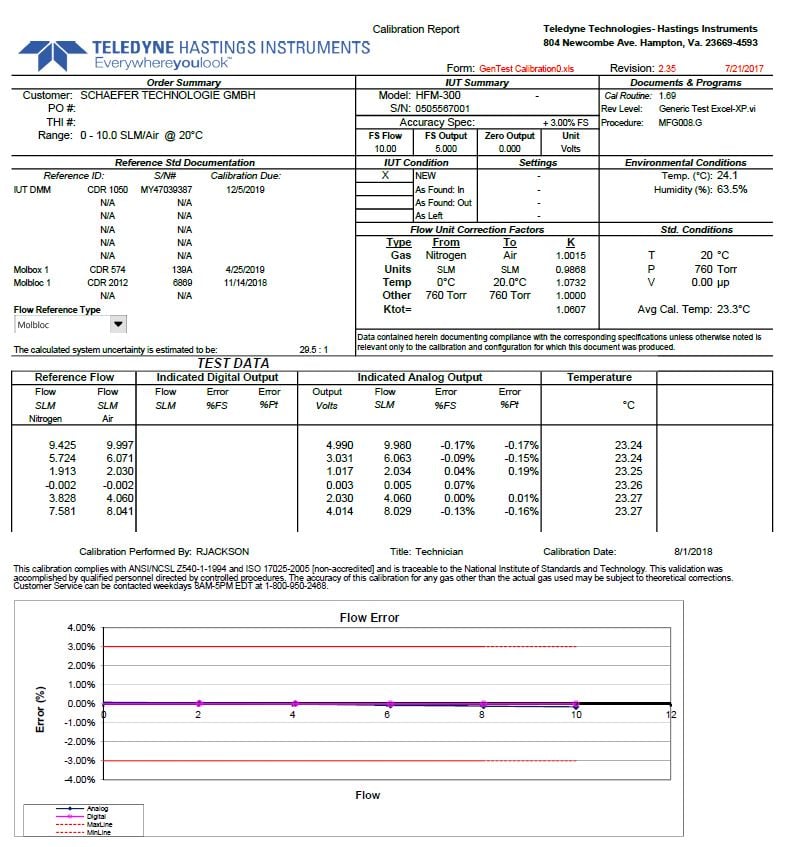
At the bottom of every one of our calibration data sheets, you will see the following statement:
This calibration complies with ANSI/NCSL Z540-1-1994 and ISO 17025-2005 [non-accredited] and is traceable to the National Institute of Standards and Technology. This validation was accomplished by qualified personnel directed by controlled procedures. The accuracy of this calibration for any gas other than the actual gas used may be subject to theoretical corrections. Customer Service can be contacted weekdays 8AM-5PM EDT at 1-800-950-2468.
Let’s start with part of the first sentence, “This calibration complies with ANSI/NCSL Z540-1-1994 and ISO 17025-2005 [non-accredited]…” According to the NCSLI webpage , there are two national standards for calibration laboratories. These are Z540-1 and ISO 17025. There are some differences between the two standards. And the aforementioned NCSLI gives a detailed description of both. In short, 17025 is appropriate for both calibration and testing labs whereas Z540-1 addresses calibration labs only. 17025 requires that the laboratory be a legal entity that can demonstrate competency, which includes thorough analysis of the uncertainty associated with the calibration services. Another difference between the two standards is that 17025 places the responsibility of the calibration due date on the end-user. In other words, the calibration lab should not determine the customers calibration cycle. That is why you no longer see calibration due dates on Teledyne Hastings’ labeling.
OK…. if 17025 is the latest, greatest, and accepted around the world, why do we still even list Z540-1 on our calibration reports? Because, we still have customers who adhere to Z540-1 and need the statement on their paperwork.
What about the word “non-accredited” that appears in parentheses? While we strive to conform to ISO 17025, which includes rigorous internal audit review, it has been our position that as a manufacturer, it is not necessary / appropriate for us to invest in the accreditation activities and third party audits. However, we do recognize the depth and critical nature of the standard. Because of those criteria, we have chosen to compose our procedures and train our personnel to be in compliance with the standard. So to be clear, Teledyne Hastings is not accredited to ISO 17025.
Let’s move on… what do we mean by, “…traceable to the National Institute of Standards and Technology”? Simply this, we can provide an unbroken chain of calibration documents that connect your calibration back to NIST, the National Institute of Standards and Technology.

Now here is a trick question… does a NIST traceable calibration tell us anything about the uncertainty of the calibration? The answer is, “no”. For example, we could calibrate one of our most advanced mass flow controllers, the HFC-D-302B 300 Vue which has a stated uncertainty of ± (0.5% of Reading + 0.2% of Full Scale).
– or we could calibrate our HFC-202 flow controller (±1% of full scale using the same metrology and the stated uncertainty for each instrument would be the same as before. In other words, the performance of these instruments does not improve just because a NIST traceable standard was used.
One more note, some customers request “Backup Documentation” to their calibration data reports. In other words, they want copies of the calibration reports of our metrology that form the unbroken link from their calibration back to NIST. There is a nominal administrative fee to collect, scan, compile, and email these calibration reports for each individual piece of metrology that was used.

Does everybody need the Backup Documentation? Usually not, but enough customers request these so it is a service that we offer. Quite often the reason why our first tier customer will request the additional supporting calibration reports is because they are manufacturing complex assemblies that their higher tier customers are procuring with the aforementioned unbroken chain back to NIST as a purchase order flow down requirement.
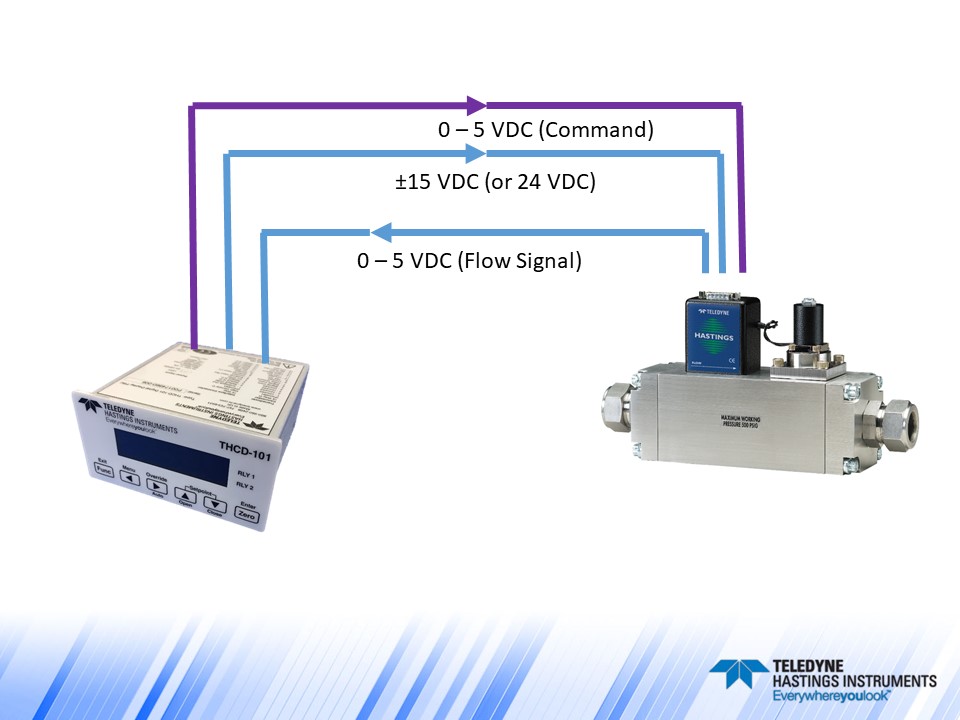
Next, we have the sentence, “This validation was accomplished by qualified personnel directed by controlled procedures” This gives us an opportunity to tell a little about our ISO 9001:2015 Quality System. As a key part of our system, all assembly and calibration personnel must complete rigorous training and demonstrate proficiency before working on either the Flow Products or Vacuum Products Teams. Also, every product or subassembly acceptance test, that has a measurable output, is controlled by a top tier Quality System Procedure. The procedures, training program, in fact the entire Quality System is subject to routine internal audit program, third party surveillance audits, and third party ISO 9001:2015 certification audits.

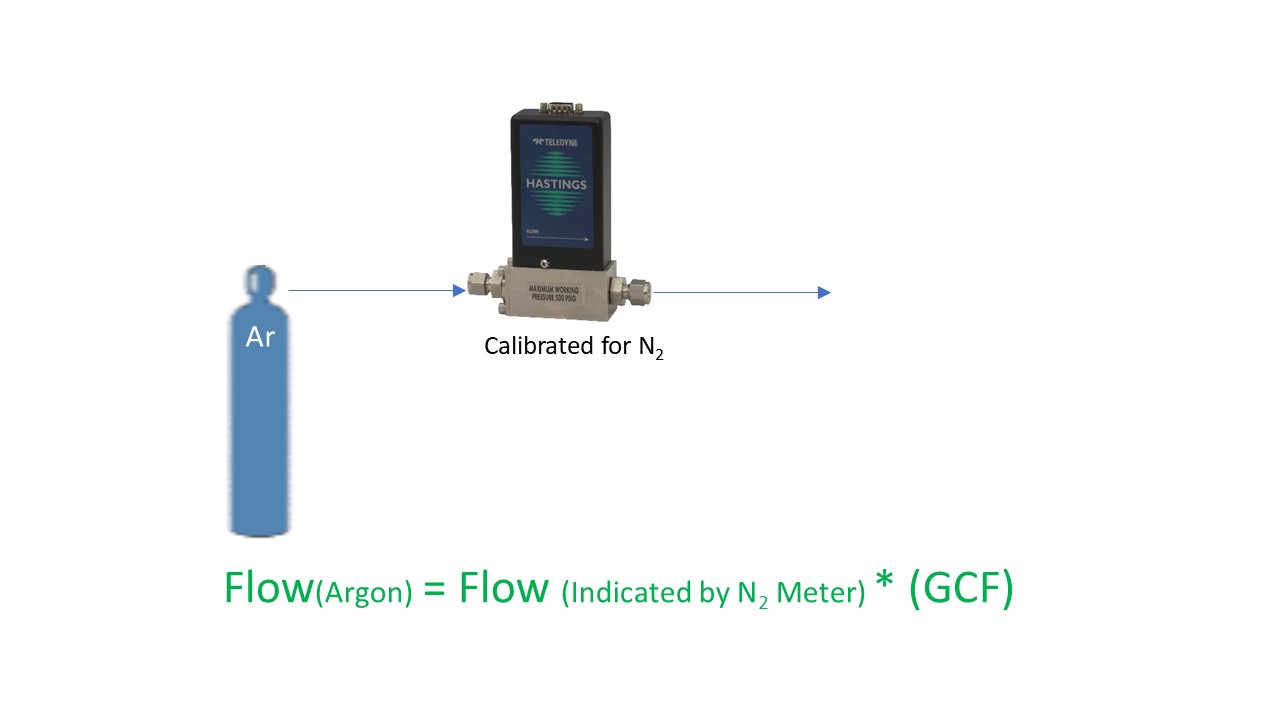
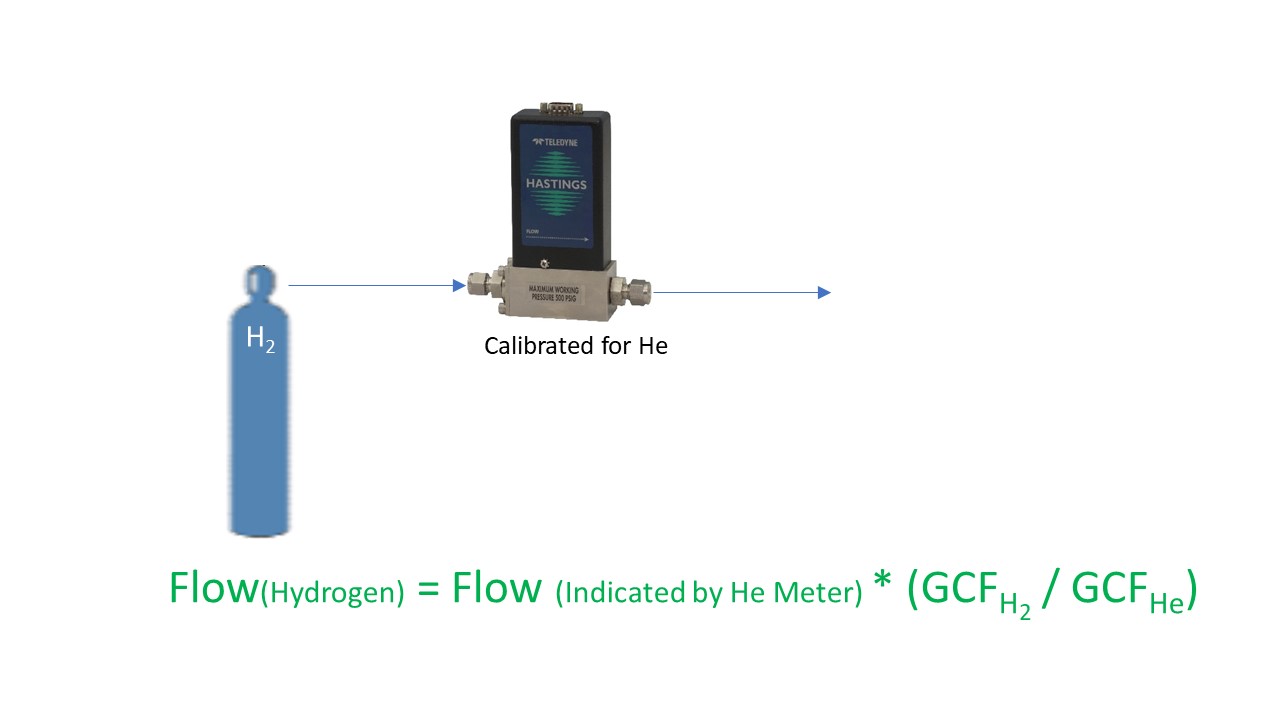
Now what about the statement, “The accuracy of this calibration for any gas other than the actual gas used may be subject to theoretical corrections”? There are certain gases which are hazardous and/or corrosive. While our flow meters and controllers are quite suitable for use in many of these gases, there are several of the gases that we have never (and will never) allow into our facility. So, we use theoretical corrections to map the output of our flow products using the calibration gas to the output for the user’s gas.
We are very proud of our metrology and quality programs. And we welcome your questions. If you have a question about mass flow controllers, vacuum gauges, or just want more information about a Calibration Report, we are here to help. You can contact us at hastings_instruments@teledyne.com or call 757-723-6531 (800-950-2468).








 The new HVG-2020B from Teledyne Hastings is a great vacuum gauge for this application. The gauge uses two vacuum sensors: a piezoresistive sensor to measure pressures from atmosphere to 10 Torr and a thermal Pirani sensor to measure from 1 Torr to 0.1 mTorr. In between 1 and 10 Torr, the gauge uses a weighted average to ensure a smooth transition between the two sensors. The piezoresistive sensor is gas species independent, so no matter what gas is being backfilled, the piezoresistive sensor gives an accurate measurement. The Pirani sensor’s response is affected by the gas species, but the user can select a gas and the correction is made.
The new HVG-2020B from Teledyne Hastings is a great vacuum gauge for this application. The gauge uses two vacuum sensors: a piezoresistive sensor to measure pressures from atmosphere to 10 Torr and a thermal Pirani sensor to measure from 1 Torr to 0.1 mTorr. In between 1 and 10 Torr, the gauge uses a weighted average to ensure a smooth transition between the two sensors. The piezoresistive sensor is gas species independent, so no matter what gas is being backfilled, the piezoresistive sensor gives an accurate measurement. The Pirani sensor’s response is affected by the gas species, but the user can select a gas and the correction is made.
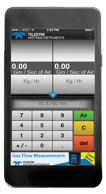 Recently, I learned that certain smartphones contain an actual pressure transducer. I shared this info with a friend who insisted that the phone was not really measuring pressure, but was instead using the internet to download the pressure based on the phone’s location. Now, I had to prove them wrong.
Recently, I learned that certain smartphones contain an actual pressure transducer. I shared this info with a friend who insisted that the phone was not really measuring pressure, but was instead using the internet to download the pressure based on the phone’s location. Now, I had to prove them wrong.
 So, it is true that you may be able to use your smartphone to measure vacuum in your system. However, we would like to suggest an easier way… check out our new
So, it is true that you may be able to use your smartphone to measure vacuum in your system. However, we would like to suggest an easier way… check out our new  This year, 2019, marks the 75th anniversary of Hastings Instruments and we will be celebrating all year long by discussing some of our past while focusing on our future. This month, I’d like to tell you a little about our glass shop.
This year, 2019, marks the 75th anniversary of Hastings Instruments and we will be celebrating all year long by discussing some of our past while focusing on our future. This month, I’d like to tell you a little about our glass shop.





 We are proud of our long history of quality craftwork, not only in the glass shop, but throughout all of our vacuum and thermal mass flow product lines here at Teledyne Hastings. The same tradition of quality goes into our newest products including the
We are proud of our long history of quality craftwork, not only in the glass shop, but throughout all of our vacuum and thermal mass flow product lines here at Teledyne Hastings. The same tradition of quality goes into our newest products including the 


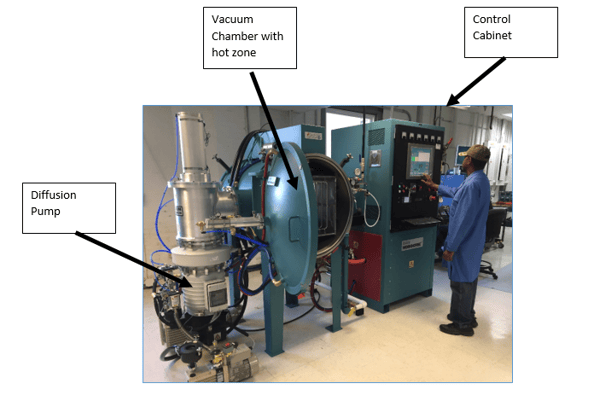
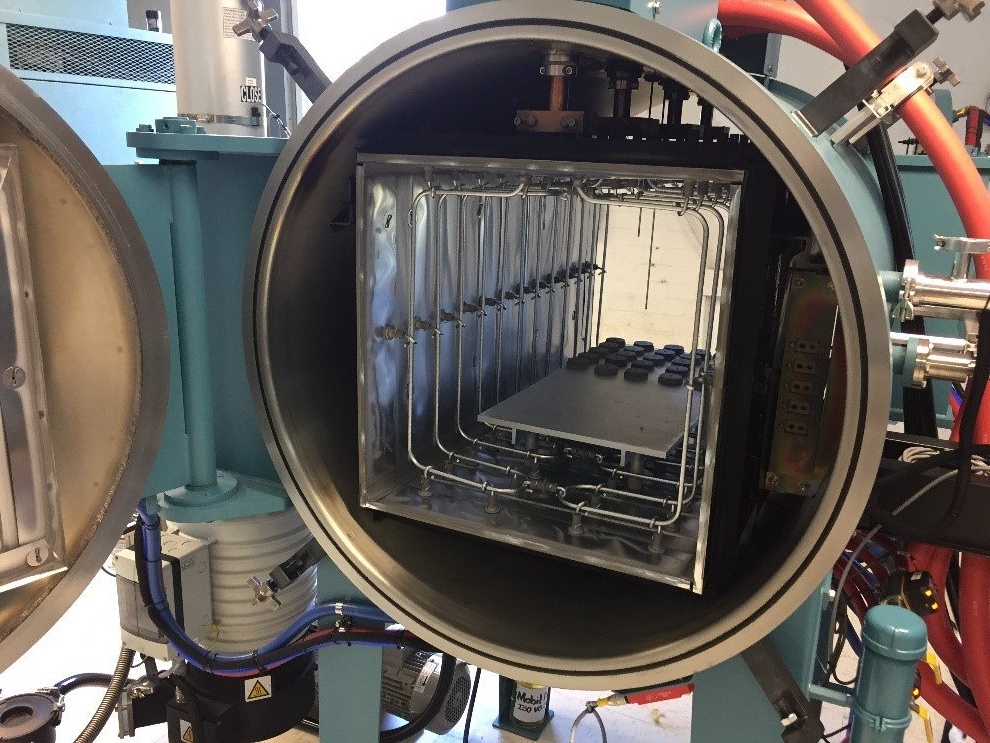 Inside the hot zone. Note the series of Molybdenum rod elements.
Inside the hot zone. Note the series of Molybdenum rod elements. Teledyne Hastings designs and build mass flow controllers for a broad array of markets from clean laboratory environments to heavy industrial installations. Recently, we have been asked to provide our newest line of Digital 300 Flow Meters and Controllers into more demanding environments. And, we are proud to offer an optional IP-67 enclosure, which provides protection against dust and water. More on our product later in the blog.
Teledyne Hastings designs and build mass flow controllers for a broad array of markets from clean laboratory environments to heavy industrial installations. Recently, we have been asked to provide our newest line of Digital 300 Flow Meters and Controllers into more demanding environments. And, we are proud to offer an optional IP-67 enclosure, which provides protection against dust and water. More on our product later in the blog. Which now brings us back to the
Which now brings us back to the 
 February is the month when citizens in the United States celebrate the history and culture of African-Americans. In early Feburary, scientists from the Pressure & Vacuum Group at NIST (National Institute of Standards & Technology) installed a special case designed to hold President Abraham Lincoln’s first handwritten draft of the Emancipation Proclamation and 13th Amendment in the Smithsonian’s National Museum of African American History & Culture. You can watch a video of the installation here:
February is the month when citizens in the United States celebrate the history and culture of African-Americans. In early Feburary, scientists from the Pressure & Vacuum Group at NIST (National Institute of Standards & Technology) installed a special case designed to hold President Abraham Lincoln’s first handwritten draft of the Emancipation Proclamation and 13th Amendment in the Smithsonian’s National Museum of African American History & Culture. You can watch a video of the installation here: Now, another interesting thing we can celebrate about the Emancipation Proclamation is the famous Emancipation Oak. Located on the campus of Hampton University, in Hampton Virginia. Note that Hampton is also the home of Teledyne Hastings. The Emancipation Oak was the site of the first reading of the Proclamation in the South according to the Hampton University Website (
Now, another interesting thing we can celebrate about the Emancipation Proclamation is the famous Emancipation Oak. Located on the campus of Hampton University, in Hampton Virginia. Note that Hampton is also the home of Teledyne Hastings. The Emancipation Oak was the site of the first reading of the Proclamation in the South according to the Hampton University Website (