Over the next several blogs, we will be discussing various industrial gases. While some of these (carbon dioxide, argon, methane, and hydrogen) may be very familiar to our readers, other gases may not be as well known. In this blog, we will take a look at sulfur hexafluoride (SF6), a gas that is one of the most important today in the utility industry.
SF6 is an interesting gas primarily because of its electrical properties. Certain neutral gas molecules can easily capture free electrons and form stable negative ions. The efficiency of negative ion formation in a gas is determined by its electron affinity. SF6 , it turns out, has a very high electron affinity and therefore has excellent electrical insulating strength. So, in an electrical discharge inside a volume containing SF6 gas, the free electrons generated by the discharge are captured by neutral SF6 to form negative ions. These large negative ions are not able to travel quickly and so the discharge is usually quickly extinguished. One other note about SF6, the insulating property of the gas improves with increasing pressure. SF6 is colorless, odorless, non-toxic, and non-flammable. As you can see, these properties make it very useful to the generation, transmission, and distribution of electricity. 80% of the world’s SF6 gas is used by electrical utilities in circuit breakers, transformers, and gas insulted switches.
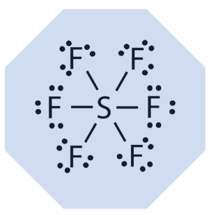
SF6 is arranged in a hexagonal structure. Each of the six fluorine atoms shares two its electrons with the outer shell of the sulfur atom in the middle. This structure gives SF6 its stability over a broad range of temperatures; the gas is thermally stable up to 500°C.

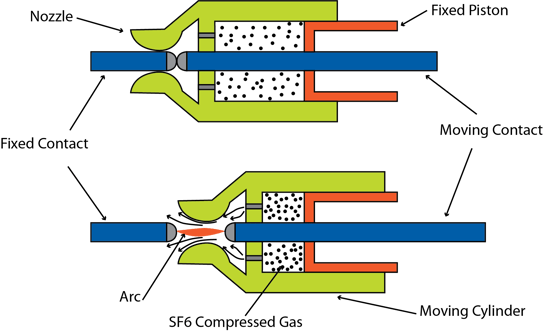
SF6 is often used in high voltage breakers. One example is the so-called dead tank breaker. In a dead tank breaker, the tank is electrically tied to earth/ground. In the live tank version, the tank is floating at a higher voltage.
The “make/break” mechanism of the breaker is shown in the diagram below. As noted above, the insulating properties of SF6 are improved with increasing pressure. So one of the jobs of the breaker’s piston actuation is to compress the SF6 gas and force it to flow into the arc region. As the contacts are moved apart, current will try to continue to flow as an arc. Any resulting arc is quickly extinguished by the pressurized SF6 flowing into the region. Incidentally, during breaker manufacturing, vacuum gauges from Teledyne Hastings are used to measure vacuum levels inside the vessel during pump down as the air is removed. After evacuation, the region can be filled with SF6.
OK, one last note about SF6 to conclude this blog and this falls under the category of “Don’t Try This at Home.” Just like Helium will make your voice sound higher if inhaled, SF6 will make your voice sound lower. You can find many demonstrations of this on YouTube. The most famous example is probably the demonstration on “The Big Bang Theory.” However, SF6 is one of the most powerful greenhouse gases and its release into the atmosphere should be minimized.

Teledyne Hastings builds both vacuum and flow instrumentation which can easily work with SF6. Note that SF6 has a very high thermal conductivity. Conceptually, this makes sense because the gas molecule has many degrees of freedom – translational, rotational, and vibrational. The GCF (gas conversion factor) for SF6 use with the 300 Vue line of flow controllers is 0.27. In other words, if you wanted to use a 300 Vue mass flow meter that had been set up for nitrogen, you would need to multiply the output by the 0.27 GCF. The good news for you is that with the 300 Vue, you can just select the gas from the front panel as shown in the photo below. Just keep in mind that if you wanted to do this, the required pressures for the valve are going to be different. You will likely need a higher pressure drop. But as always, our application engineers can be reached by email, phone, or Live Chat on our website: www.teledyne-hi.com
