Note: The content of this blog was updated February 4, 2023 to provide more information for the reader.
The applications engineers here at Teledyne Hastings discussed topics for our blog. We all agreed that one of the more frequent questions fielded, involves the units used to measure vacuum levels. We find that the technicians who use their vacuum system daily often seem to develop a sixth sense about the “health” of their systems. They know something isn’t quite right when the base vacuum pressure (or rate of pressure change) is not what they expect. So, when vacuum pressure measurements are inconsistent from batch-to-batch, that is the time when the user stops to ask the meaning behind the data that their vacuum measurement instrumentation is providing.
Measuring Vacuum Pressures
A vacuum exists when there is negative pressure, or when there is system pressure that is less than atmospheric pressure. Manufacturing processes generate different levels of vacuum when operating at peak efficiency for a given vacuum application that are measured using a vacuum gauge. Absolute vacuum is the absence of all matter. Atmospheric pressure, also known as barometric pressure, is the pressure due to earth’s atmosphere. Atmospheric pressure is 760 Torr or 14.696 psia at sea level and changes with altitude. The vacuum pressure scale is book-ended by absolute vacuum pressure in the “ultra-high” vacuum range and atmospheric pressure at the “rough” vacuum range. It should be noted that absolute vacuum, or perfect vacuum, is never truly attained.
Most users know that vacuum is commonly measured using units of pressure. There are a few different systems of pressure measurement, and this blog will discuss those most used. In Armand Berman’s book, Total Pressure Measurements in Vacuum Technology, pressure unit systems are divided into two categories: “Coherent Systems” and “Other Systems”.
Common Units of Vacuum
“Coherent Systems” of units are based on the definition of pressure (P) as the force (F) exerted on a chamber wall per unit area (A). P = F/A. The International System of Units, or SI units, is commonly used for pressure measurement. http://physics.nist.gov/cuu/Units/units.html The SI unit for pressure is the Pascal (Pa), and it is interesting to note that NIST (National Institute of Standards and Technology) published papers are always required to use SI units. Again, the SI unit for pressure (force per unit area) is the Pascal. 1 Pa = 1 N /m2.
As a unit of pressure, the Pascal is not always convenient to use because vacuum systems often operate in pressure ranges where collected data results in large numbers. For example, near atmospheric pressure, we would measure approximately 100,000 Pa. So, a more convenient unit, the bar, was developed. (1 bar = 100,000 Pa)
Moving lower in pressure, it is very helpful to then use the mbar (1 mbar = 0.001 bar), which has become the predominate unit of measure in Europe, as the basis for describing pressure levels. As a specific example, look at the base pressure specification of a turbo pump, which will typically be given in terms of mbar (e.g., Base Pressure < 1 x 10-10 mbar).
“Other Systems” of units include the Torricelli system, which is based on an experiment (shown in the diagram below) conducted by the Italian scientist, Evangelista Torricelli. In this experiment, the pressure exerted on the mercury can be shown to be P = hdg, where h is the height of the mercury column, d is the density, and g is the acceleration due to gravity.

By measuring the mercury column height, the pressure can be determined. The Torr unit (named after Torricelli) has been defined as 1 millimeter of mercury (1 Torr = 1 mmHg). This unit, as well as the use of the mTorr unit (1 mTorr = 0.001 Torr), is commonly used in the United States. Historically, pressure was sometimes described in terms of “microns”, which simply meant a mercury column height of one micron (1x10-6 m). Note that the micron and the mTorr are the same.
Lastly, it should be noted that occasional confusion arises between the use of different, but seemingly similar, units of pressure. As explained above, the mbar and the mTorr are not the same. One mbar has the same order of magnitude as one Torr (1 mbar = 0.75 Torr).
Unit Conversions
The table below gives some conversion values between various commonly used units of pressure and vacuum. A useful website for conversions:
http://www.onlineconversion.com/pressure.htm
| |
Pa
|
mbar
|
Torr
|
mTorr (micron)
|
Atm
|
|
1 Pa =
|
1
|
0.01
|
0.0075
|
7.50
|
~ 10-5
|
|
1 mbar =
|
100
|
1
|
-.75
|
750.06
|
~ 10-3
|
|
1 Torr =
|
133.3
|
1.333
|
1
|
1000.0
|
~ 10-3
|
|
1 mTorr (micron) =
|
0.1333
|
0.00133
|
0.001
|
1
|
~ 10-6
|
|
1 Atm =
|
101,325
|
1013.25
|
760
|
760,000
|
1
|
Absolute Pressure and Gauge Pressure
In conclusion, keep in mind that vacuum measurements can be referenced to ambient pressure, gauge pressure measurement or absolute pressure measurement (perfect vacuum). An absolute pressure measurement is referenced with respect to absolute vacuum. Absolute pressure will often be designated by the letter “a” after the unit of measure; “psia.” As an example, an absolute pressure reading of 30 psia (pounds per square inch absolute) is a pressure that is 30 psi above vacuum. It is important to understand that there is no negative absolute pressure. There are some
Gauge pressure measurements are measured relative to the ambient atmospheric pressure. Relative, or gauge pressure, will often be designated by the letter “g” after the unit of measure; “psig.” As an example, 30 psig is a gauge pressure that is 30 psi above ambient atmosphere (typically 14.7 psia at sea level). In this example the gauge pressure, 30 psig, is equal to an absolute pressure of 44.7 psia.
What is a vacuum system?
A vacuum system can consist of multiple a vacuum pumps and vacuum gauges attached to a tank that is designed to measure below atmospheric pressure. The vacuum pumps reduce the air pressure inside of the tank to the pressure range that the vacuum pump is rated for. Different vacuum pumps bring the vacuum pressure to different levels depending on the strength of the vacuum pump.
Attached to the tank is usually at least one vacuum pressure gauge. An application may require another type of vacuum gauge to measure a different pressure point. An example of this would be using a piezo pressure gauge and a Pirani pressure gauge to have a larger range of the vacuum pressure measured. A piezo vacuum gauge would be used to measure the rough vacuum range around atmosphere and the Pirani could be used to measure below 1 Torr in the mTorr range. There are vacuum gauges that use technologies from different vacuum gauges to create a combination vacuum gauge to measure vacuum pressure across a wider pressure range. Teledyne Hastings combines both the Pirani and piezo technologies to make the HVG-2020B vacuum pressure gauge.
Vacuum Gauges Poster
 |
Vacuum Gauges
Free Poster
Unit Conversions and More

|
Original Content posted Sept 22, 2014
FAQ Corner - Units for Vacuum Measurement
an overview of units used to measure pressure
Earlier this year, the applications engineers here at Teledyne Hastings discussed topics for our blog. We all agreed that one of the more frequent questions that we discuss with folks involve the units used to measure vacuum levels. We find the technicians who use their vacuum systems daily often seem to develop a sixth sense about the “health” of their systems. They know something isn’t quite right when the base pressure (or rate of pressure change) is not what they expect. So, when pressure measurements are not consistent from batch to batch, that is the time when the user stops to ask the meaning behind the data that their vacuum measurement instrumentation is providing.
Now, most users know that vacuum is commonly measured using units of pressure. There are a few different sets of pressure units, and this blog will discuss the more commonly used ones. In Armand Berman’s book, Total Pressure Measurements in Vacuum Technology, pressure unit systems are divided into two categories: “Coherent Systems” and “Other Systems”.
Coherent Systems of Units are based on the definition of pressure (P) as the force (F) exerted on a chamber wall per unit area (A). P = F/A. The International System of Units, or SI units, is commonly used for pressure measurement. http://physics.nist.gov/cuu/Units/units.html The SI unit for pressure is the Pascal (Pa). It interesting to note that at NIST (National Institute of Standards and Technology), published papers are always required to use the SI set of units. Again, the SI unit for pressure (force per unit area) is the Pascal. 1 Pa = 1 N /m2.
Now, the Pascal as a unit of pressure is not always the most convenient because vacuum systems are often operating in a range of pressures where we would need to collect data using large numbers. For example, near atmospheric pressure, we would measure approximately 100,000 Pa. So, a more convenient unit, the bar, has been derived. (1 bar = 100,000 Pa)
Moving lower in pressure, it is very helpful to then use the mbar (1 mbar = 0.001 bar). So many vacuum users, especially in Europe, use the mbar as the basis for describing pressure levels. As a specific example, look at the base pressure specification of a turbo pump, it will be given in terms of mbar (e.g. Base Pressure < 1 x 10-10 mbar).
Another system of pressure units is based on the Torricelli experiment (shown in the diagram). In this experiment, the pressure exerted on the mercury can be shown to be P = hdg, where h is the height of the mercury column, d is the density, and g is the acceleration due to gravity.
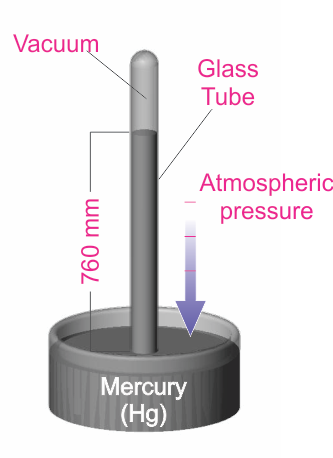
By measuring the mercury column height, the user can determine the pressure. The Torr unit (named for the Italian scientist Torricelli) has been defined to be 1 millimeter of mercury (1 Torr = 1 mmHg). This unit is very common, especially in the United States. It is also common to use the mTorr (1 mTorr = 0.001 Torr). Many years ago, pressure was sometimes described in terms of “microns”, which simply meant a mercury column height of one micron (1x10-6 m). Note that the micron and the mTorr are the same.
One last word about the units used to measure vacuum: on occasion, there is confusion between pressure units. As we have seen above, the mbar and the mTorr are not the same. One mbar has the same order of magnitude as one Torr (1 mbar ≈ 0.75 Torr). The table below gives some approximate conversion values. A useful website for conversions:
http://www.onlineconversion.com/pressure.htm
|
|
Pa
|
mbar
|
Torr
|
mTorr (micron)
|
Atm
|
|
1 Pa =
|
1
|
0.01
|
0.0075
|
7.50
|
~ 10-5
|
|
1 mbar =
|
100
|
1
|
0.75
|
750.06
|
~ 10-3
|
|
1 Torr =
|
133.3
|
1.333
|
1
|
1000.0
|
~ 10-3
|
|
1 mTorr (micron) =
|
0.1333
|
0.00133
|
0.001
|
1
|
~ 10-6
|
|
1 Atm =
|
101,325
|
1013.25
|
760
|
760,000
|
1
|
Douglas Baker is the Director of Sales & Business Development of Teledyne Hastings. Antonio Araiza prepared the Torricelli experiment drawing. Antonio is the head of Technical Documentation at Teledyne Hastings (and is among the best soccer referees in the Commonwealth of Virginia).
 March is Women’s History Month and this year we’d like to focus on Mary Hastings, one of the key founders of Hastings Instruments. Mary Comstock graduated from William & Mary with a degree in physics with minors in math and chemistry. She was truly a pioneer in many ways. After college, she took a job as a “computer” at NACA (National Advisory Committee for Aeronautics) in Hampton, Virginia. There, Mary met Charles Hastings, a young engineer who had his office across the hall from her. The two were married in 1940.
March is Women’s History Month and this year we’d like to focus on Mary Hastings, one of the key founders of Hastings Instruments. Mary Comstock graduated from William & Mary with a degree in physics with minors in math and chemistry. She was truly a pioneer in many ways. After college, she took a job as a “computer” at NACA (National Advisory Committee for Aeronautics) in Hampton, Virginia. There, Mary met Charles Hastings, a young engineer who had his office across the hall from her. The two were married in 1940.

 The early part of the 1950’s was prosperous for Hastings due in part to the demand for the Raydist and large military contracts as a result of the Korean War. Sales nearly tripled between 1950 and 1953 and there were almost 200 employees. Hastings had outgrown its space yet again and expanded to a 14,000 square foot building on Newcomb Avenue (current day location for Teledyne Hastings). The building was originally used as a car barn for street cars, then as a World War I armory and eventually as a manufacturing plant for ladies clothing.
The early part of the 1950’s was prosperous for Hastings due in part to the demand for the Raydist and large military contracts as a result of the Korean War. Sales nearly tripled between 1950 and 1953 and there were almost 200 employees. Hastings had outgrown its space yet again and expanded to a 14,000 square foot building on Newcomb Avenue (current day location for Teledyne Hastings). The building was originally used as a car barn for street cars, then as a World War I armory and eventually as a manufacturing plant for ladies clothing.
 A small percentage of Hastings business during the early 1950’s was for instrument sales. The most important of these products were the air-meters, vacuum gauges, flow meters, accelerometers and an electronic standard cell. In order to grow this part of the business, Hastings decided to set up a manufacturer’s representative program. By the end of 1953, Hasting’s was looking forward to seeing this manufacturer’s representative program vastly increasing instrument sales.
A small percentage of Hastings business during the early 1950’s was for instrument sales. The most important of these products were the air-meters, vacuum gauges, flow meters, accelerometers and an electronic standard cell. In order to grow this part of the business, Hastings decided to set up a manufacturer’s representative program. By the end of 1953, Hasting’s was looking forward to seeing this manufacturer’s representative program vastly increasing instrument sales.
 By 1947, the Hastings Instrument Company could count many successful projects. Their list of products included the following:
By 1947, the Hastings Instrument Company could count many successful projects. Their list of products included the following: After several sales pitches and demonstrations, Hastings received two large contracts for Raydist. Along with these two contracts, the company was busy building Air-Meters for commercial sales. Before selling the Air-Meters, the instruments needed to be calibrated. In those early days, calibration was done by driving down the road holding a probe out the window while someone in the passenger seat held the Air-Meter. When the car reached 5, 10, 15 etc… mph the passenger would make a note on the blank dial face and then return to the house where they would neatly letter the dial face.
After several sales pitches and demonstrations, Hastings received two large contracts for Raydist. Along with these two contracts, the company was busy building Air-Meters for commercial sales. Before selling the Air-Meters, the instruments needed to be calibrated. In those early days, calibration was done by driving down the road holding a probe out the window while someone in the passenger seat held the Air-Meter. When the car reached 5, 10, 15 etc… mph the passenger would make a note on the blank dial face and then return to the house where they would neatly letter the dial face. During this period of growth, Hastings realized that it was time to find a new location for the business. By now, there were 17 people working elbow-to-elbow at the Hastings’ home and that could not continue. The company settled on temporary location in an old brick distributorship building that had a leaky roof and flooded at spring tides, but it was at the price they could afford.
During this period of growth, Hastings realized that it was time to find a new location for the business. By now, there were 17 people working elbow-to-elbow at the Hastings’ home and that could not continue. The company settled on temporary location in an old brick distributorship building that had a leaky roof and flooded at spring tides, but it was at the price they could afford.
 I
I n addition to the list of commercial instruments above, Hastings developed specialized instruments for specific customers. For example: the “Ventimeter” was used by the army to measure ventilation in clothing to keep wearers comfortable under extreme weather conditions. The Hastings Company was now growing fast and generating handsome profits for its stakeholders.
n addition to the list of commercial instruments above, Hastings developed specialized instruments for specific customers. For example: the “Ventimeter” was used by the army to measure ventilation in clothing to keep wearers comfortable under extreme weather conditions. The Hastings Company was now growing fast and generating handsome profits for its stakeholders.
 In September 1944, the Hastings Instrument Company started to take shape. For quite some time, Charles & Mary conducted the business out of their home. They received their first order in December from the Naval Aircraft Factory in Philadelphia for $800. The order was for a rotary magnetic switch for commutating electrical circuits.
In September 1944, the Hastings Instrument Company started to take shape. For quite some time, Charles & Mary conducted the business out of their home. They received their first order in December from the Naval Aircraft Factory in Philadelphia for $800. The order was for a rotary magnetic switch for commutating electrical circuits.  Business continued to grow. Seventeen employees would arrive at the Hastings home around 7pm on Monday, Wednesday and Friday to work on their electronic projects (see image on right). During the day, Mary would take care of miscellaneous projects. On one occasion, Mary agreed to have some Raydist cabinets painted by the time Charles came home. Unfortunately, the air compressor was out of air so Mary came up with another plan. She would take the car to the nearby service station and put as much air in the tires as she could without them bursting. She would then drive back home, attach her paint sprayer to the tires, and paint the Raydist cabinets
Business continued to grow. Seventeen employees would arrive at the Hastings home around 7pm on Monday, Wednesday and Friday to work on their electronic projects (see image on right). During the day, Mary would take care of miscellaneous projects. On one occasion, Mary agreed to have some Raydist cabinets painted by the time Charles came home. Unfortunately, the air compressor was out of air so Mary came up with another plan. She would take the car to the nearby service station and put as much air in the tires as she could without them bursting. She would then drive back home, attach her paint sprayer to the tires, and paint the Raydist cabinets  until her tires were almost flat. She did this several times to complete the project before Charles came home. The business activities took a toll on the Hastings home. The roof leaked and needed to be replaced from all the antennas mounted to it (see image on left), the driveway needed to be replaced from the damage of delivery trucks, Mary’s oven smelled like paint which caused some challenges when meal time came.
until her tires were almost flat. She did this several times to complete the project before Charles came home. The business activities took a toll on the Hastings home. The roof leaked and needed to be replaced from all the antennas mounted to it (see image on left), the driveway needed to be replaced from the damage of delivery trucks, Mary’s oven smelled like paint which caused some challenges when meal time came. In January 1946, Hastings received their first order for a Raydist. The Air Material Command at Wright Field in Cleveland Ohio wanted a single-dimensional Raydist system to use during aerial photography and mapping. The final product was hand-delivered by Charles himself in October. (see image on right and below)
In January 1946, Hastings received their first order for a Raydist. The Air Material Command at Wright Field in Cleveland Ohio wanted a single-dimensional Raydist system to use during aerial photography and mapping. The final product was hand-delivered by Charles himself in October. (see image on right and below)
 Ever wonder where the idea or dream of Hastings originated? Well as part 1 of our anniversary year blog posts, we thought this would be a good place to start. Charles Hastings at the age of 10 was bitten by the radio bug and began to build and experiment with radio gear. In 1930, at the age of 16, Charles Hastings found an opportunity to fund his experiments by fixing other people’s radios. Many families had radios at this point, but they were very unreliable and frequently needed minor repairs. Charles would fix radios to earn money to buy parts for his own experiments.
Ever wonder where the idea or dream of Hastings originated? Well as part 1 of our anniversary year blog posts, we thought this would be a good place to start. Charles Hastings at the age of 10 was bitten by the radio bug and began to build and experiment with radio gear. In 1930, at the age of 16, Charles Hastings found an opportunity to fund his experiments by fixing other people’s radios. Many families had radios at this point, but they were very unreliable and frequently needed minor repairs. Charles would fix radios to earn money to buy parts for his own experiments. Last month, we passed our ISO 9001 surveillance audit. It has been over twenty years since we first obtained ISO and we wanted to take a step back and review some significant accomplishments.
Last month, we passed our ISO 9001 surveillance audit. It has been over twenty years since we first obtained ISO and we wanted to take a step back and review some significant accomplishments. 






 Pittcon is an annual conference on laboratory science that is organized by The Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy. Pittcon started as a small technical conference held in 1950. The first 18 conferences were held in Pittsburgh, Pennsylvania, but the conference has since grown. Locations now vary from year to year with this year’s conference being held at the Pennsylvania Convention Center in Philadelphia, Pennsylvania from March 17-21.
Pittcon is an annual conference on laboratory science that is organized by The Pittsburgh Conference on Analytical Chemistry and Applied Spectroscopy. Pittcon started as a small technical conference held in 1950. The first 18 conferences were held in Pittsburgh, Pennsylvania, but the conference has since grown. Locations now vary from year to year with this year’s conference being held at the Pennsylvania Convention Center in Philadelphia, Pennsylvania from March 17-21.